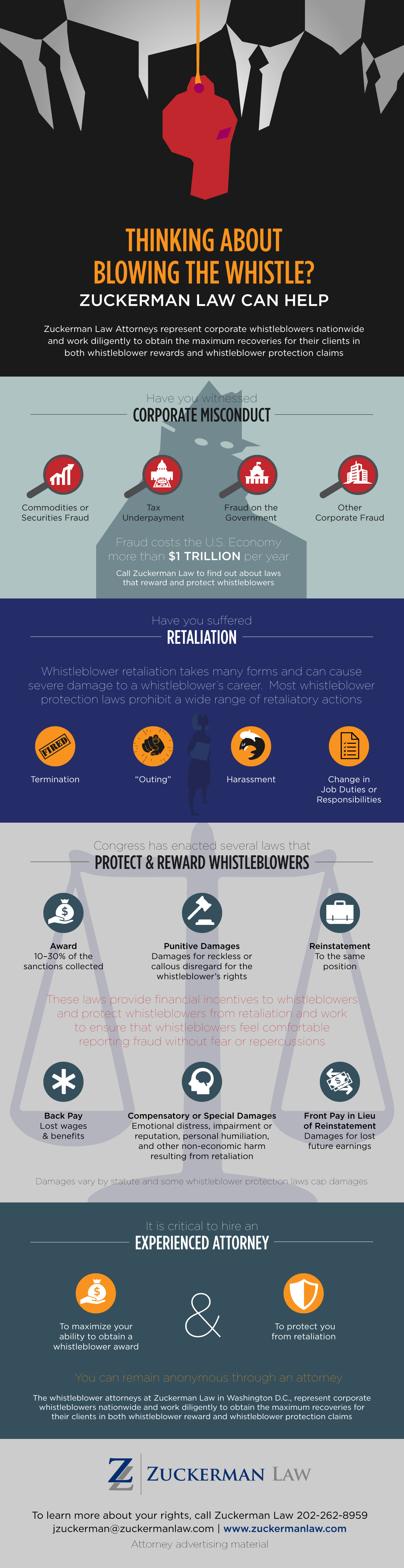
False Claims Prohibited by the False Claims Act







The False Claims Act prohibits:
- knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval; and
- knowingly making, using, or causing to be made or used, a false record or statement material to a false or fraudulent claim.
To prevail, a qui tam whistleblower must prove that:
- the defendant submitted a claim to the government;
- the claim was false; and
- the defendant knew the claim was false.
Express Legal Falsity (Factually False Claim)
A claim of express falsity arises where a contractor fails to comply with the requirements for the goods or services that it agreed to provide the federal government. A factually false claim is one that “is untrue on its face,” for example if it “include[s] ‘an incorrect description of goods or services provided or a request for reimbursement for goods or services never provided.’” United States v. Kellogg Brown & Root Servs., Inc., 800 F. Supp. 2d 143, 154 (D.D.C. 2011) (citing United States v. Sci. Applications Int’l Corp. (SAIC II), 626 F.3d 1257, 1266 (D.C. Cir.2010)). Examples include billing for services that were never provided or charging the government for an armored vehicle but providing a vehicle that is not armored.
The FCA defines “material” as “having a natural tendency to influence, or be capable of influencing, the payment or receipt of money or property.” 31 U.S.C. § 3729(b)(4). Escobar held that to properly plead materiality, a plaintiff must show that the effect or likely behavior of the government—if it knew that the defendant had made false statements in seeking payment—would be to refuse payment. Id. at 2002. “The materiality standard is demanding” because the FCA “is not an all-purpose antifraud statute or a vehicle for punishing garden-variety breaches of contract or regulatory violations.” Id. at 2003.
A claim for reimbursement from Medicare is false when it “does not comply with statutory conditions for payment,” such as the Medicare statutory requirement that the items and services claimed are “reasonable and necessary.” United States ex rel. Petratos v. Genentech, 855 F.3d 481, 487 (3d Cir. 2017) (quoting 42 U.S.C. § 1395y(a)(1)(A)). See also 42 C.F.R. § 411.15(k)(l). Under the Medicare statute, “no payment may be made . . . for any expenses incurred for items or services . . . [that] are not reasonable and necessary for the diagnosis or treatment of illness or injury.” 42 U.S.C. § 1395w-102(e)(3); 42 U.S.C. § 1395y(a)(l)(A) (emphasis added). Notwithstanding FDA approval, a claim must involve a drug that is “‘reasonable and necessary for [the] individual patient’ based on ‘accepted standards of medical practice and the medical circumstances of the individual case.’” Id. (alteration and emphasis in original) (quoting Medicare Benefit Policy Manual, ch. 15, § 50.4.3). There are instances “when a drug treatment could be approved by the FDA and used for a medically accepted indication, but still not be ‘reasonable and necessary’” for an individual patient. Id.
Legal Falsity (False Certification)
A false certification may be either express or implied:
- Express false certification occurs when a claimant explicitly represents that he or she has complied with a statute, regulation, or contractual term, but in fact has not complied.
- Implied false certification occurs when “the defendant submits a claim for payment that makes specific representations about the goods or services provided, but knowingly fails to disclose the defendant’s noncompliance with a statutory, regulatory, or contractual requirement,” and that “omission renders those representations misleading.” Escobar, 136 S. Ct. at 1995.
A claim of implied certification arises where the claim for payment to the Government implicitly constitutes certification of compliance with certain applicable regulations. A government contractor’s non-compliance with a government regulation can violate the False Claims Act where there is a relevant connection to the contract at issue. In 2016, the Supreme Court held in Escobar that an FCA complaint premised on implied certification must satisfy “two conditions”: “first, the claim . . . makes specific representations about the goods or services provided; and second, the defendant’s failure to disclose non-compliance with material statutory, regulatory, or contractual requirements makes those representations misleading half-truths.”
Escobar also provides important guidance on materiality:
- Materiality turns on the “effect on the likely or actual behavior of the recipient of the alleged misrepresentation.” Universal Health, 136 S. Ct. 1989 at 2002.
- To plead materiality with the requisite particularity, a relator may draw inferences from various sources, including the Government’s history of declining to pay claims for failure to comply with the applicable regulation. See Universal Health, 136 S. Ct. at 2003 (noting that materiality may be premised on “evidence that the defendant knows that the Government consistently refuses to pay claims in the mine run of cases based on noncompliance with the particular statutory, regulatory, or contractual requirement[s]”).
- Materiality is absent at the pleading stage when the relator’s chronology suggests that the Government knew of the alleged fraud, yet paid the contractor anyway. See Universal Health, 136 S. Ct. at 2003-04 (“[I]f the Government pays a particular claim in full despite its actual knowledge that certain requirements were violated, that is very strong evidence that those requirements are not material. Or, if the Government regularly pays a particular type of claim in full despite actual knowledge that certain requirements were violated, and has signaled no change in position, that is strong evidence that the requirements are not material.”).
The difference between express certification and implied certification is whether the entity seeking payment must certify that it has complied with the applicable law, rule, or regulation each time a claim is made, or if that certification is made initially and later implied with each subsequent claim.
Fraud-In-The-Inducement or Promissory Fraud
The False Claims Act also prohibits fraud-in-the-inducement, i.e., where the contract or extension of government benefit was originally obtained through false statements or fraudulent conduct.
The Supreme Court recognized a fraud-in-the-inducement theory when it held in U.S. ex. rel. Marcus v. Hess, 317 U.S. 537 (1943) that contracts obtained under a collusive bidding scheme violated the FCA by defrauding the government and compelling it to pay more “than it would have been required to pay had there been free competition in the open market.”
To establish fraudulent inducement under the FCA, a relator must show that a false statement, omission, or misrepresentation “`caused’ or `induced’ the government to enter into a contract, such that but for the misrepresentations, the government would not have awarded the contract and would not have paid the claim.” United States ex rel. Thomas v. Siemens AG, 991 F. Supp. 2d 540, 569 (E.D. Pa. 2014).
In United States ex rel. Campie v. Gilead Scis., Inc., 862 F.3d 890 (9th Cir. 2017), the court found that allegations that a drug manufacturer’s claims for reimbursement for its drugs from the government were sufficient to allege falsity because the manufacturer allegedly gained approval of the drugs through false statements to the FDA. In that case, a “fail[ure] to disclose the defendant’s violation of a material statutory, regulatory, or contractual requirement . . . renders the claim ‘false and fraudulent.'” Id. at 901 (quoting Universal Health Servs. v. United States ex rel. Escobar, 136 S. Ct. 1989, 1995 (2016)). An implied certification claim must satisfy two conditions to succeed: “first, the claim does not merely request payment, but also makes specific representations about the goods or services provided; and second, the defendant’s failure to disclose noncompliance with material statutory, regulatory, or contractual requirements makes those representations misleading half-truths.” Escobar, 136 S. Ct. at 2001.
A Grant Assurance is a Claim
A grant assurance in an application for federal funds or a grant progress report is a “claim” under the False Claims Act since representations made in the progress report trigger the payment of grant funds. See United States ex rel. Bauchwitz v. Holloman, 671 F.Supp.2d 674, 689 (E.D.Pa.2009).
Experienced False Claims Act Whistleblower Lawyers







Described by the National Law Journal as a “leading whistleblower attorney,” founding Principal Jason Zuckerman has established precedent under a wide range of whistleblower protection laws and obtained substantial compensation for his clients and recoveries for the government in whistleblower rewards and whistleblower retaliation cases. Three of the cases he worked on are featured in Tom Mueller’s seminal book about whistleblowing Crisis of Conscience: Whistleblowing in an Age of Fraud and Dan Maldea’s Corruption in U.S. Higher Education: The Stories of Whistleblowers. The False Claims Act qui tam cases that Zuckerman has worked on in conjunction with other attorneys have resulted in recoveries in excess of $100 million.
2022 False Claims Act Settlements
| Amount | Violations | Date | Source |
|---|---|---|---|
| $900M | Biogen Inc. agreed to pay $900 million to resolve allegations that it violated the FCA by paying kickbacks to physicians to induce them to prescribe the company’s multiple sclerosis drugs. The relator alleged that Biogen held programs through which it offered and paid remuneration, including speaker honoraria, speaker training fees, consulting fees and meals, to health care professionals who spoke at or attended Biogen’s speaker programs, speaker training meetings or consultant programs to induce them to prescribe the drugs Avonex, Tysabri and Tecfidera in violation of the Anti-Kickback Statute. | September 26, 2022 | Biogen Inc. Agrees to Pay $900 Million to Settle False Claims Act Allegations Related to Improper Physician Payments |
| $260M | Mallinckrodt resolved allegations that it violated the FCA by knowingly: 1) underpaying Medicaid rebates due for its drug H.P. Acthar Gel; and 2) using a foundation as a conduit to pay illegal co-pay subsidies in violation of the Anti-Kickback Statute for Acthar. | March 7, 2022 | Mallinckrodt Agrees to Pay $260 Million to Settle Lawsuits Alleging Underpayments of Medicaid Drug Rebates and Payment of Illegal Kickbacks |
| $61M | A jury ordered Eli Lilly and Co. to pay $61 million in damages for false claims about pricing to Medicaid programs to lower rebates that it owed to the states. | August 2022 | Jury orders Lilly to pay $61M in whistleblower Medicaid fraud case |
| $48.5M | In the largest-ever False Claims Act recovery based on allegations of small business contracting fraud, TriMark agreed to pay $48.5 million to resolve allegations that its subsidiaries, TriMark Gill Marketing and Gill Group, Inc. improperly manipulated federal small business set-aside contracts around the country. TriMark identified federal set-aside contract opportunities for the small businesses to bid on using their set-aside status; instructed them regarding how to prepare their bids and what prices to propose; “ghostwrote” emails for those companies to send to government officials to make it appear as though the small businesses were performing work that TriMark was performing; and affirmatively concealed TriMark’s involvement in the contract. | February 23, 2022 | Government Contractor Agrees to Pay Record $48.5 Million to Resolve Claims Related to Fraudulent Procurement of Small Business Contracts Intended for Service-Disabled Veterans |
| $45M | BioTelemetry, Inc. agreed to pay $44.875 million to settle allegations that it defrauded U.S. taxpayers by outsourcing critical remote medical services to technicians in India who were not properly trained. | December 21, 2022 | Philadelphia-area Company To Pay $45 Million Whistleblower Settlement For Outsourcing Heart Monitoring To India |
| $45M | Modernizing Medicine Inc., an EHR technology vendor, agreed to pay $45 million to resolve allegations that it violated the FCA by accepting and providing unlawful remuneration in exchange for referrals and by causing its users to report inaccurate information in connection with claims for federal incentive payments. | November 1, 2022 | Modernizing Medicine Agrees to Pay $45 Million to Resolve Allegations of Accepting and Paying Illegal Kickbacks and Causing False Claims |
| 38.5M | Academy Mortgage Corporation agreed to pay $38.5 million to resolve allegations it violated the False Claims Act by improperly originating and underwriting mortgages insured by the Federal Housing Administration. | December 14, 2022 | Academy Mortgage Corporation Agrees to Pay $38.5 Million to Settle False Claims Act Allegations Related to Mortgages Insured by the Federal Housing Administration |
| $40M | Relator Simpson alleged that Bayer paid kickbacks to hospitals and physicians to induce them to utilize the drugs Trasylol and Avelox, and also marketed these drugs for off-label uses that were not reasonable and necessary. Simpson further alleged that Bayer downplayed the safety risks of Trasylol. Simpson also filed a second lawsuit alleging that Bayer knew about, but downplayed, Baycol’s risks of causing rhabdomyolysis. | September 2, 2022 | Bayer to Pay $40 Million to Resolve the Alleged Use of Kickbacks and False Statements Relating to Three Drugs |
| $34M | Eargo agreed to pay $34.37 million to resolve allegations that it submitted or caused the submission of claims for hearing aid devices for reimbursement to the Federal Employees Health Benefits Program (FEHBP) that contained unsupported hearing loss diagnosis codes. | April 29, 2022 | Hearing Aid Company Eargo Inc. Agrees to Pay $34.37 Million to Settle Common Law and False Claims Act Allegations for Unsupported Diagnosis Codes |
| $24.5M | Physician Partners of America LLC (PPOA), its founder, and its former chief medical officer agreed to pay $24.5 million to resolve allegations that they violated the FCA by billing federal healthcare programs for unnecessary medical testing and services, paying unlawful remuneration to its physician employees and making a false statement in connection with a loan obtained through the PPP. | April 12, 2022 | Physician Partners of America to Pay $24.5 Million to Settle Allegations of Unnecessary Testing, Improper Remuneration to Physicians and a False Statement in Connection with COVID-19 Relief Funds |
| $24M | Respironics, Inc. agreed to pay over $24 million to settle an FCA qui tam alleging improper inducement to DME suppliers. The government and the relator alleged that the defendant violated the Anti-Kickback Statute when it provided DME suppliers with free physician prescribing data for its marketing efforts to physicians. The purported illegal inducement allegedly caused the DME suppliers to submit false claims for respiratory-related medical equipment. | September 1, 2022 | Philips Subsidiary to Pay Over $24 Million for Alleged False Claims Caused by Respironics for Respiratory-Related Medical Equipment |
| $22.9M | CHC Holdings, LLC d/b/a Carter Healthcare, an Oklahoma limited liability company that provides home healthcare through subsidiaries in multiple states, as well as Stanley Carter and Brad Carter agreed to pay $22,948,004 to resolve allegations that Carter Healthcare wrongfully paid physicians to induce referrals of home health patients under the guise of medical directorships, resulting in the submission of false claims to the Medicare and TRICARE programs. | October 18, 2022 | Oklahoma City Home Health Company and Two Former Corporate Officers Agree to Pay $22.9 Million to Settle Federal False Claims Act and Kickback Allegations Arising From Improper Payments to Referring Physicians |
| $22.5M | Dignity Health agreed to pay $22.5 million pursuant to two separate settlements to resolve allegations that they violated the FCA and CA FCA by causing the submission of false claims to Medi-Cal related to Medicaid Adult Expansion under the ACA. | December 7, 2022 | Three Health Care Providers Agree to Pay $22.5 Million for Alleged False Claims to California’s Medicaid Program |
| $20M | BayCare Health System Inc. and entities that operate four affiliated Florida hospitals (collectively BayCare) have agreed to pay the United States $20 million to resolve allegations that BayCare violated the False Claims Act by making donations to the Juvenile Welfare Board of Pinellas County (JWB) to improperly fund the state’s share of Medicaid payments to BayCare. The four hospitals are Morton Plant Hospital, Mease Countryside Hospital, Mease Dunedin Hospital and St. Anthony’s Hospital. Specifically, the United States alleged that during this time, BayCare made improper, non-bona fide cash donations to JWB knowing that JWB would and then did transfer a portion of the cash donations to the State of Florida’s Agency for Health Care Administration for Florida’s Medicaid Program. The funds transferred by JWB to the state were “matched” by the federal government before being returned to the BayCare hospitals as Medicaid payments, and BayCare was thus able to recoup its original donations to JWB and also receive federal matching funds, in violation of the federal prohibition on non-bona fide donations. BayCare’s donations to JWB increased Medicaid payments received by BayCare, without any actual expenditure of state or local funds. | April 6, 2022 | Florida’s BayCare Health System and Hospital Affiliates Agree to Pay $20 Million to Settle False Claims Act Allegations Relating to Impermissible Medicaid Donations |
| $14.6M | Massachusetts General Hospital, the clinical teaching arm for Harvard Medical School, resolved a federal whistleblower case stemming from allegations that some of the hospital's orthopedic surgeons engaged in overlapping surgeries that violated federal Medicare and Commonwealth of Massachusetts Medicaid rules. | February 19, 2022 | MASS GENERAL HOSPITAL TO PAY $14.6 MILLION TO RESOLVE OVERLAPPING SURGERY CLAIMS; STANDARDIZED CONSENT FORMS TO BE AMENDED |
| $14M | Georgia Cancer Specialists, agreed to pay $8 million to resolve allegations that GCS solicited and received kickbacks for more than a decade, first from Option Care, an infusion pharmacy and medical equipment provider, and later from Amedisys, a Medicare nursing company. The whistleblowers received $2.4 million dollars, the maximum possible relators’ share. | February 1, 2022 | Louis J. Cohen, Whistleblower Counsel, Announces Georgia Cancer Specialists Agrees to Pay $8 Million Dollars to Resolve Medicare Fraud Kickback and Stark Law Violations; Total Settlements Exceed $14 Million |
| $13M | Cardinal Health agreed to pay $13,125,000 to resolve allegations that it violated the False Claims Act by paying “upfront discounts” to its physician practice customers, in violation of the Anti-Kickback Statute. | January 31, 2022 | Cardinal Health Agrees to Pay More than $13 Million to Resolve Allegations that it Paid Kickbacks to Physicians |
| $12.95M | Medical device manufacturer Biotronik agreed to pay $12.95 million to resolve allegations that it violated the FCA by paying kickbacks to physicians to induce their use of Biotronik’s implantable cardiac devices, such as pacemakers and defibrillators. | July 22, 2022 | Medical Device Manufacturer Biotronik Inc. Agrees To Pay $12.95 Million To Settle Allegations of Improper Payments to Physicians |
| $12.6M | Advanced Bionics LLC, manufacturer of cochlear implant system devices, agreed to pay more than $12 million to resolve allegations that it misled federal health care programs regarding the radio-frequency emission generated by some of its devices. More specifically, Advanced Bionics is alleged to have manipulated testing conditions to obtain passing test results by not testing processors in “worst-case” configurations, and improperly shielding certain emissions-generating components of the cochlear implant system during emissions testing – all contrary to the standard’s requirements. | December 20, 2022 | Advanced Bionics LLC to Pay Over $12 Million for Alleged False Claims for Cochlear Implant Processors |
| $9.85 | BioReference agreed to pay $9.85 million to resolve alleged violations of the FCA arising from BioReference’s payment of above-market rents to physician landlords for office space to induce referrals to BioReference. | July 14, 2022 | BioReference Laboratories and Parent Company Agree to Pay $9.85 Million to Resolve False Claims Act Allegations of Illegal Payments to Referring Physicians |
| $9M | Aerojet Rocketdyne Holdings Inc. and whistleblower Brian Markus settled a False Claims Act suit alleging that the company misled the government about its cybersecurity practices to gain missile defense and rocket engine contracts. | April 29, 2022 | Aerojet Rocketdyne, Whistleblower Settle Cybersecurity Suit |
| $7.9M | Akorn agreed to pay $7.9 million to settle a qui tam action alleging fraudulent billing of Medicare. The government and the relator alleged that the FDA approved of a prescription only ("Rx-only") to over the counter ("OTC") status conversion for three brand name drugs. The defendant allegedly made generic equivalents of the drugs, which would require it to seek FDA approval for OTC status or seek withdrawal of the products' Rx-only status and halt marketing. This purportedly caused Medicare Part D to pay for products ineligible for coverage. | September 14, 2022 | Pharmaceutical Company Akorn Operating Company LLC Agrees to Pay $7.9 Million to Resolve Allegations of Fraudulent Billing |
| $7.4M | Six surgery centers and medical offices affiliated with Interventional Pain Management Center P.C. settled a qui tam action for mischaracterizing acupuncture as a surgical procedure in order to dishonestly obtain millions of dollars from Medicare and the Federal Employees Health Benefit Program. The defendants treated patients with electro-acupuncture devices called P-Stim and NeuroStim/NSS (“NSS”). P-Stim and NSS procedures transmit electrical pulses through needles placed just under the skin on a patient’s ear. Both treatments are considered acupuncture under Medicare and Federal Employees Health Benefit Program (“FEHBP”) guidelines and are therefore ineligible for reimbursement by the government. From January 2012 through April 2017, the IPMC surgery centers and medical offices submitted claims to Medicare and FEHBP for P-Stim and NSS treatment and associated administration of anesthesia. In submitting the claims, the defendants used a billing code that mischaracterized the acupuncture treatment as a surgical implantation of a neurostimulator. | January 12, 2022 | Surgery Centers and Medical Offices in New Jersey Settle Allegations of Federal Health Care Fraud |
| $6.85M | YRC Freight Inc, Roadway Express Inc. and Yellow Transportation Inc. agreed to pay approximately $6.85 million to resolve allegations that they knowingly presented false claims to DOD by systematically overcharging for freight carrier services and making false statements to hide their misconduct. | March 14, 2022 | Freight Carriers Agree to Pay $6.85 Million to Resolve Allegations of Knowingly Presenting False Claims to the Department of Defense |
| $6.8M | DermaTran Health Solutions, LLC; Pharmacy Insurance Administrators, LLC; Legends Pharmacy; TriadRx; and the former owners of Lake Side Pharmacy agreed to pay $6,8M to resolve allegations that they violated the FCA by waiving copays, charging the government higher prices than permitted, and trading federal healthcare business with other pharmacies. | October 12, 2022 | DermaTran and three other pharmacies pay over $6.8 million to settle civil claims |
| $5.5M | American Senior Communities, L.L.C. agreed to pay approximately $5.5M to resolve allegations that it violated the FCA by charging Medicare directly for various therapy services provided to beneficiaries who had been placed on hospice, when those services should have already been covered by the beneficiaries’ Medicare hospice coverage. | August 10, 2022 | U.S. Attorney’s Office Recovers Over $5.5 Million in Civil False Claims Settlement with American Senior Communities |
| $4.2M | The government alleges that DOCS and Sidana submitted false claims to Medicare and Medicaid for immunotherapy services that were not medically necessary, and were not directly supervised by a physician. The allegations also involve claims to Medicare and Medicaid for medically unnecessary annual re-testing of allergy patients. | December 15, 2022 | Connecticut Physician and Urgent Care Practice Pay Over $4.2 Million to Settle False Claims Act Allegations |
| $4.5M | Northern Arizona Healthcare, Flagstaff Medical Center and Health First Foundation agreed to pay a total of $4.5 million to settle allegations that a 2017 payment to FMC under the Medicaid Disproportionate Share Hospital program violated federal law. The relator alleged that a Medicaid DSH Pool 5 payment to FMC in 2017 violated the federal prohibition against non-bona fide provider-related donations. | August 29, 2022 | Northern Arizona Healthcare, Flagstaff Medical Center and Health First Foundation – Northern Arizona Agree to Pay $4.5 Million to Settle False Claims Act Lawsuit |
| $4M | Philips North America LLC agree to pay $4.2 million to settle claims it swapped out key components of a mobile patient monitoring device that it sold to the U.S. military without recertifying the device for airworthiness, allegedly putting top government officials, first responders and the military at risk. | August 31, 2022 | Philips To Pay $4M Over Claims It Skirted Military Testing Rule |
| $2.1M | SHC Home Health Services of Florida, LLC and its related entities (collectively “Signature HomeNow”) paid $2.1 million to the United States government to settle claims of improperly billing the Medicare Program for home health services provided to beneficiaries living in Florida. The complaint alleged that Signature HomeNow knowingly submitted false or fraudulent claims seeking payment from the Medicare Program for home health services to Medicare beneficiaries who: (i) were not homebound; (ii) did not require certain skilled care; (iii) did not have a valid or otherwise appropriate plans of care in place; and/or (iv) did not have appropriate face-to-face encounters needed in order to be appropriately certified to receive home health services. | May 5, 2022 | Home Health Company Operating in Florida Pays $2.1 Million to Resolve False Claims Allegations |
| $2M | Hayat Pharmacy agreed to pay approximately $2M to resolve allegations that it submitted false claims to Medicare and Medicaid in 2019 for two prescription medications and switched Medicaid and Medicare patients from lower cost medications to the iodoquinol-hydrocortisone-aloe cream and Azesco without any medical need and/or without a valid prescription. | January 28, 2022 | Milwaukee Pharmacy Chain to Pay Over $2 Million to Resolve Allegations It Violated the False Claims Act |
| $1.2M | Philips RS North America LLC, formerly known as Respironics, Inc., a nationwide manufacturer of sleep and respiratory durable medical equipment, agreed to pay $1,283,825.40 to settle allegations that it unlawfully induced referrals for its equipment in violation of the False Claims Act and Anti-Kickback Statute. | September 22, 2022 | Sleep and Respiratory Equipment Manufacturer to Pay $1.2 Million to Resolve Allegations of Unlawful Kickbacks |
| $930,000 | Comprehensive Health Services, LLC agreed to pay $930,000 to resolve allegations that it violated the False Claims Act by falsely representing to the State Department and the Air Force that it complied with contract requirements relating to the provision of medical services at State Department and Air Force facilities in Iraq and Afghanistan. The United States alleged that, between 2012 and 2019, CHS failed to disclose to the State Department that it had not consistently stored patients’ medical records on a secure EMR system. | March 8, 2022 | Contractor Pays $930,000 to Settle False Claims Act Allegations Relating to Medical Services Contracts at State Department and Air Force Facilities in Iraq and Afghanistan |
| $800,000 | Dr. Bergman and his medical practice agreed to pay $800,000 to the US and Iowa to resolve allegations that Bergman wrongfully billed Medicare and Medicaid for services rendered by others and billed Medicare for medically unnecessary and unreasonable applications of skin substitute products. | September 7, 2022 | Iowa Plastic Surgeon Agrees to Pay $800,000 to Resolve Allegations of Inappropriate Billing and False Claims |
False Claims Act Qui Tam Whistleblower Awards

- What is a qui tam whistleblower lawsuit?
- What types of false claims are prohibited by the False Claims Act?
- What is the first-to-file bar in False Claims Act qui tam cases?
- What is the requirement to file a False Claims Act qui tam action under seal?
- Are False Claims Act whistleblowers protected against retaliation?
- What is a reverse false claim?
- What is the statute of limitations for a False Claims Act qui tam action?
- What is the public disclosure bar in the False Claims Act?
- What is the original source exception to the public disclosure bar?
- What is materiality under the False Claims Act?
- What is “Scienter” Under the False Claims Act?
- Is a Violation of the Anti-Kickback Law Also a Violation of the False Claims Act?
- Does the False Claims Act Prohibit Bid-Rigging?
- Does the False Claims Act Prohibit Fraudulent Inducement of a Contract?
- Can a violation of Good Manufacturing Practices give rise to False Claims Act Liability?
- Is there a heightened pleading requirement for False Claims Act qui tam cases?
- Does the False Claims Act authorize treble damages?
- Must a False Claims Act qui tam relator have firsthand knowledge of all aspects of the fraud?